
Determine the enthalpy of the ATP reaction.
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Activity/Lab
- Provider:
- Carnegie Mellon University
- Provider Set:
- The ChemCollective
- Date Added:
- 02/05/2021

Determine the enthalpy of the ATP reaction.
In this activity, students use the virtual lab to create 500mL of 3M HCl solution from a concentrated stock solution of 11.6M HCl. They must first calculate the correct volumes of 11.6M HCl solution and water to mix together to create the final solution. Next, they prepare the solution using the appropriate glassware, and then can check their answer using the concentration viewer in the solution info panel.
Determine the concentration of an alcohol solution from its density.
You probably have a general understanding of how your body works. But do you fully comprehend how all of the intricate functions and systems of the human body work together to keep you healthy? This course will provide that insight. By approaching the study of the body in an organized way, you will be able to connect what you learn about anatomy and physiology to what you already know about your own body.
By taking this course, you will begin to think and speak in the language of the domain while integrating the knowledge you gain about anatomy to support explanations of physiological phenomenon. The course focuses on a few themes that, when taken together, provide a full view of what the human body is capable of and of the exciting processes going on inside of it.
Topics covered include: Structure and Function, Homeostasis, Levels of Organization, and Integration of Systems.
Note: This free course requires registration
Apply the sampling distribution of the sample mean as summarized by the Central Limit Theorem (when appropriate). In particular, be able to identify unusual samples from a given population.
In this part of the MRE scenario, students measure the enthalpy of a reaction.
In this part of the MRE scenario, students determine change in the enthalpy of a reaction as the concentration of reactants are varied.

Interview with Anita Newell
Word Count: 6138
(Note: This resource's metadata has been created automatically as part of a bulk import process by reformatting and/or combining the information that the author initially provided. As a result, there may be errors in formatting.)
Use the virtual lab to determine how much milk to add to hot coffee to reach the desired temperature
In this activity, students use the virtual lab to prepare a sucrose solution for a soda recipe. They next calculate the concentration of their solution in terms of molarity, percent mass and density.
The Virtual Lab is an online simulation of a chemistry lab. It is designed to help students link chemical computations with authentic laboratory chemistry. The lab allows students to select from hundreds of standard reagents (aqueous) and manipulate them in a manner resembling a real lab.
Simulations:
• Default Virtual Lab Stockroom
• Stoichiometry
• Glucose Dilution Problem
• Acid Dilution Problem
• Cola and Sucrose Concentration Problem
• Making Stock Solutions from Solids
• Identifying the Unknown Metal (Metals Density Problem)
• Identifying an Unknown Liquid from its Density
• Alcohol Density Problem
• Gravimetric Determination of Arsenic
• Determining Stoichiometric Coefficients
• Stoichiometry and Solution Preparation Problem
• Textbook Style Limiting Reagents Problems
• Textbook Style Limiting Reagents Problem II
• Predicting DNA Concentration
• Unknown Concentration of DNA Solution Problem
• Thermochemistry
• Camping Problem I
• Camping Problem II
• ATP Reaction (Thermochemistry and Bonding)
• Determining the Heat of Reaction in Aqueous Solution
• Coffee Problem
• Measuring the heat capacity of an engine coolant
• Measuring the heat capacity of an engine coolant II (Advanced version)
• Camping Problem III
• Heats of Reaction - Hess' Law
• Equilibrium
• Cobalt Chloride and LeChatlier’s Principle
• DNA Binding Problem
• Acid-Base Chemistry
• Strong Acid and Base Problems
• Determination of the pH Scale by the Method of Successive Dilutions
• Weak Acid and Base Problems
• Determining the pKa and Concentration Ratio of a Protein in Solution
• Unknown Acid and Base Problem
• Creating a Buffer Solution
• DNA - Dye Binding: Equilibrium and Buffer Solutions
• Determining the pKa and Concentration Ratio of a Protein in Solution
• Standardization of NaOH with a KHP solution: Acid Base Titration
• Solubility
• Determining the Solubility Product
• Temperature and the Solubility of Salts
• Determining the solubility of copper chloride at different temperatures
• Oxidation/Reduction and Electrochemistry
• Exploring Oxidation-Reduction Reactions
• Analytical Chemistry/Lab Techniques
• Standardization of NaOH with a KHP solution: Acid Base Titration
• Unknown Silver Chloride
In this activity, students perform an experiment to determine the heat of a reaction.
Learning Objectives: 1).Determine point estimates in simple cases, and make the connection between the sampling distribution of a statistic, and its properties as a point estimator.
2). Explain what a confidence interval represents and determine how changes in sample size and confidence level affect the precision of the confidence interval.
3). Find confidence intervals for the population mean and the population proportion (when certain conditions are met), and perform sample size calculations.
1). Summarize and describe the distribution of a categorical variable in context.
2). Generate and interpret several different graphical displays of the distribution of a quantitative variable (histogram, stemplot, boxplot).
3). Summarize and describe the distribution of a quantitative variable in context: a) describe the overall pattern, b) describe striking deviations from the pattern.
4). Relate measures of center and spread to the shape of the distribution, and choose the appropriate measures in different contexts.
5). Compare and contrast distributions (of quantitative data) from two or more groups, and produce a brief summary, interpreting your findings in context.
5). Apply the standard deviation rule to the special case of distributions having the "normal" shape.
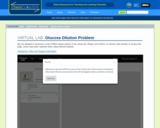
In this activity, students use the virtual lab to create a 0.025M glucose solution from a standard 1M glucose solution. First, they calculate the correct volumes of 1M glucose solution and water to mix together to create the final 0.025M solution. Next, they prepare the solution using the appropriate glassware. Students can check to see if their procedure was correct using the concentration viewer in the solution info panel.
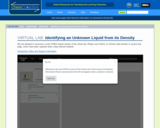
In this activity students use the virtual lab to design an experiment to determine the identity of mislabeled bottles using the densities of the solutions inside.
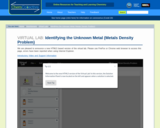
In this activity, students use the virtual lab to identify an unknown metal by measuring its density and comparing their measurements to the densities of known metals.
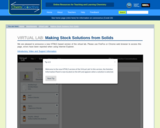
In this activity, students use the virtual lab to create stock solutions starting from solid salts. Students must first calculate the correct amount of solid to make the solution. Next, they prepare the solution using the appropriate glassware. Students can check to see if their procedure was correct using the concentration viewer in the solution info panel.
You and a friend are hiking the Appalachian Trail when a storm comes through. You stop to eat, but find that all available firewood is too wet to start a fire. From your Chem 106 class, you remember that heat is given off by some chemical reactions; if you could mix two solutions together to produce an exothermic reaction, you might be able to cook the food you brought along for the hike. Luckily, being the dedicated chemist that you are, you never go anywhere without taking along a couple chemical solutions called X and Y just for times like this. The Virtual Lab contains solutions of compounds X and Y of various concentrations.
As an analytical chemist at a company developing new engine coolants your task is to determine the heat capacity of a newly developed product and then to determine if its heat capacity is greater of less than that of ethylene glycol.