Introductory Astronomy for Undergraduates
Light, Energy and Atoms
Light is so important in the field of Astronomy because the light that is reflected off of planets, stars, etc is the reason why we are able to identify celestial bodies that are light years away. Astronomers are constantly discovering new planets and stars in the sky and that can often be attributed to the fact that the light from that planet or star has finally reached Earth. We will never know about some planets and stars because it will take a very long time for the light of that planet or star to travel all the way to the Earth to allow us to see it at all.
Light is considered an important tool in astronomy because it helps us see things. For example, in order for us to see an object there must be light present. In astronomy where telescopes and other tools are used to see objects in space, light is needed to see these objects more clearly. Telescopes collect more light than our eyes can in order for us to see things we would normally not be able to see. The study of electromagnetic waves has shaped modern life through technology. We have learned how to use these waves to create and use things such as cell phones, radio, televisions and remotes, and laptops. Basically everything we use on a daily basis require the use of electromagnetic waves.
Introduction to electromagnetic waves
Electromagnetic radiation is one of the many ways that energy travels through space. The heat from a burning fire, the light from the sun, the X-rays used by your doctor, as well as the energy used to cook food in a microwave are all forms of electromagnetic radiation. While these forms of energy might seem quite different from one another, they are related in that they all exhibit wavelike properties.
If you’ve ever gone swimming in the ocean, you are already familiar with waves. Waves are simply disturbances in a particular physical medium or a field, resulting in a vibration or oscillation. The swell of a wave in the ocean, and the subsequent dip that follows, is simply a vibration or oscillation of the water at the ocean’s surface. Electromagnetic waves are similar, but they are also distinct in that they actually consist of 2 waves oscillating perpendicular to one another. One of the waves is an oscillating magnetic field; the other is an oscillating electric field.
Please read from the source:https://www.khanacademy.org/science/physics/light-waves/introduction-to-light-waves/a/light-and-the-electromagnetic-spectrum
The electromagnetic spectrum
Electromagnetic waves can be classified and arranged according to their various wavelengths/frequencies; this classification is known as the electromagnetic spectrum. The following table shows us this spectrum, which consists of all the types of electromagnetic radiation that exist in our universe.
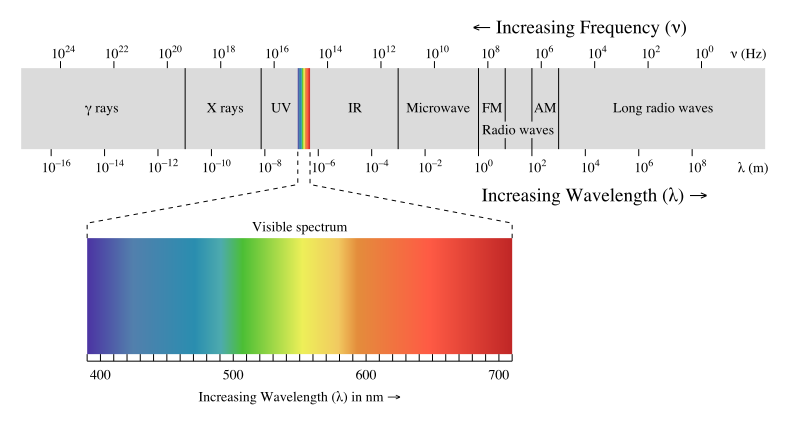
The electromagnetic spectrum Image from UC Davis ChemWiki
The
electromagnetic spectrum is comprised of all the varieties of radiation
in the universe. Gamma rays have the highest frequency, whereas radio
waves have the lowest. Visible light is approximately in the middle of
the spectrum, and comprises a very small fraction of the overall
spectrum.
The electromagnetic spectrum. Image from UC Davis ChemWiki, CC-BY-NC-SA 3.0
The Structure of the Atom
Atoms have been theorised to exist since the days of the ancient Greeks, when the philosopher Democritus realised that at a certain point, he could not cut his bread any smaller: it was indivisible. He suggested that everything must be made up of some fundamental blocks, which cannot be destroyed, and he named them after the Greek word ‘atomos’, meaning ‘no cut’.
Just like bricks are the building blocks of a home, atoms are the building blocks of matter. Matter is anything that has mass and takes up space (volume). All matter is made up of atoms. The atom has a nucleus, which contains particles of positive charge (protons) and particles of neutral charge (neutrons). Surrounding the nucleus of an atom are shells of electrons - small negatively charged particles. These shells are actually different energy levels and within the energy levels, the electrons orbit the nucleus of the atom
When electrons move from a higher energy level to a lower one, photons are emitted, and an emission line can be seen in the spectrum. Absorption lines are seen when electrons absorb photons and move to higher energy levels. Since each atom has its own characteristic set of energy levels, each is associated with a unique pattern of spectral lines. This allows astronomers to determine what elements are present in the stars and in the clouds of gas and dust among the stars. An atom in its lowest energy level is in the ground state. If an electron is in an orbit other than the least energetic one possible, the atom is said to be excited. If an atom has lost one or more electrons, it is called an ion and is said to be ionized. The spectra of different ions look different and can tell astronomers about the temperatures of the sources they are observing[1].