Circadian Rhythms
Circadian Rhythms
Adapted from “Kimball’s biology pages”
Bud is six years old and super excited, because he normally goes to bed by 8:00pm and is asleep by 8:30pm, but tonight is New Year’s Eve. He is allowed to stay up to enjoy the festivities! Bud’s mom makes him some hot chocolate and they put on his favorite movie at 8:00pm. Maybe he can watch another one before midnight! He is happy and content as the story unfolds. He snuggles in, next to his mom. But then, to his dismay, his eyes begin to close! It’s just a little after 9:00. He sits up straight and his mom brings him some water. To his horror he nods off again! Maybe it’s the movie - he picks out another that is even more exciting than the last. It’s a new one that he has been waiting to watch. He sits upright, eyes glued to the screen... and wakes up the next morning in his bed. What happened?
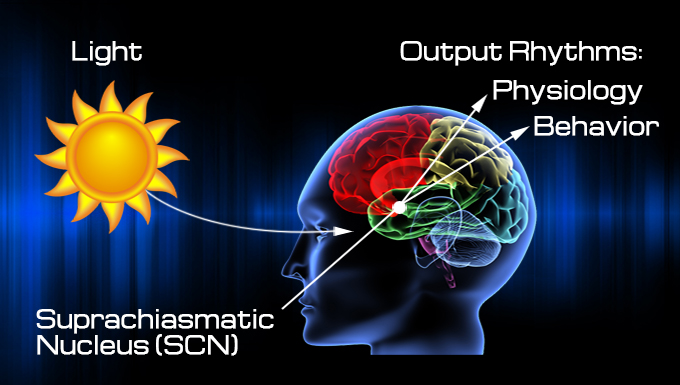
Bud fell victim to his circadian rhythms, a biological oscillation with a time period of about 24 hours. Circadian rhythms are found in every species, waxing and waning with the minutes and hours of the day. While each cell in your body has its own timepiece that runs on a molecular feedback loop, each rhythm is coordinated by the suprachiasmatic nucleus (SCN) - two paired structures sitting just above the roof of your mouth that keep internal organs and their processes synchronized to each other and to the external environment. Our rhythms uphold patterns in sleeping and eating; they influence our ability to rise early or stay up late, and to adjust to changes in time zones and shift work. Alterations in rhythms may influence our susceptibility to disease, productivity at work, and attention.
In this chapter you will learn:
- the defining characteristics of circadian rhythms
- the molecular machinery that runs cellular rhythms in mammals and insects
- the neural structures that regulate and coordinate our behavioral expression of circadian rhythmicity
- the role of circadian rhythms in health and disease.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Glossary
entrained → to determine or modify the phase or period of circadian rhythms
zeitgeber → external or environmental cue that entrains or synchronizes an organism’s biological rhythms
period/tau → length of a biological rhythm
PRC → phase response curve; describes the relationship between a stimulus (such as light exposure) and a response (such as a shift in circadian rhythm)
diurnal → describes organisms that are active during the day and sleep at night
nocturnal → describes organisms that are active at night and sleep during the day
circadian rhythm → biological process that displays an endogenous, entrainable oscillation of roughly 24 hours
phase → series of biological events
freerunning → sleep pattern that is neither entrained to a 24 hour cycle in nature nor an artificial cycle
infradian rhythm → a biological rhythm that is repeated longer than a 24 hour circadian day and therefore recurs over a longer period than a circadian rhythm
ultradian rhythm → recurrent period or cycle less than 24 hours
Circadian Clocks
We live in a rhythmic world. The earth turns on its axis, presenting a new day every 24 hours. The earth also revolves around the sun, creating dramatic changes in daylength and temperature that we refer to as seasons. The moon waxes and wanes, tides come and go, all with a predictable period. These predictable patterns are routinely used by all living organisms as a matter of survival to predict changes in light and temperature. For example, diurnal animals (those that are awake during the day) return to their burrows before the night sets in. This allows them to avoid nocturnal predators who are much better equipped to find them in the dark. Tidal clocks allow marine invertebrates to synchronize their reproductive behavior to insure procreation. Circannual rhythms prepare species for drastic changes in food availability, temperature, and landscape from season to season. Thus, biological rhythms vary in frequency and can be classified by period length. Those with periods greater than a day are referred to as infradian, and those with periods less than 24 hours are referred to as ultradian. The most ubiquitous and well known are those with periods of about 24 hours, or circadian (circa - about; dian = day).
True biological rhythms are driven by internal oscillating systems. As the environment we live in also oscillates, it is often difficult to determine if the rhythm we are observing is an active process (endogenously driven) or a passive response to external stimuli (hourglass). There are aspects of oscillating systems that distinguish those driven endogenously from the others.
Characteristics of Circadian Rhythms
Circadian rhythms are a subset of biological rhythms that are characterized by three factors:
- Circadian rhythms are endogenous. When the organism is placed in constant conditions (e.g., continuous darkness), these rhythms persist or freerun. Circadian rhythms have a period close to, but not exactly, 24 hours, giving rise to the name circadian rhythms. Without environmental cues, circadian rhythms tend to be somewhat longer or somewhat shorter than 24 hours.
- Circadian rhythms can be synchronized or entrained by zeitgeber signals (light, food, activity) that occur with regular frequency. There are limits to the period length that a zeitgeber can set. For circadian rhythms, the period is no greater than 22-26 hours. For example, circadian rhythms will NOT entrain to a zeitgeber with an 18 hour rhythm. Light is the most powerful zeitgeber - one second of bright light can synchronize wheel running rhythms of laboratory rodents[1]. Other zeitgebers include access to food[2], exercise[3], and drugs[4].
- Circadian rhythms are temperature compensated. That is, they are independent of changes in the organism’s internal temperature. This fundamental property is important because the ambient temperature changes over the course of the day and the seasons of the year. A temperature-sensitive clock would slow down at lower temperatures and speed up at higher temperatures, making the clock unreliable. While many rhythms have been shown to maintain their periods in vivo and in vitro, the mechanism is unknown.
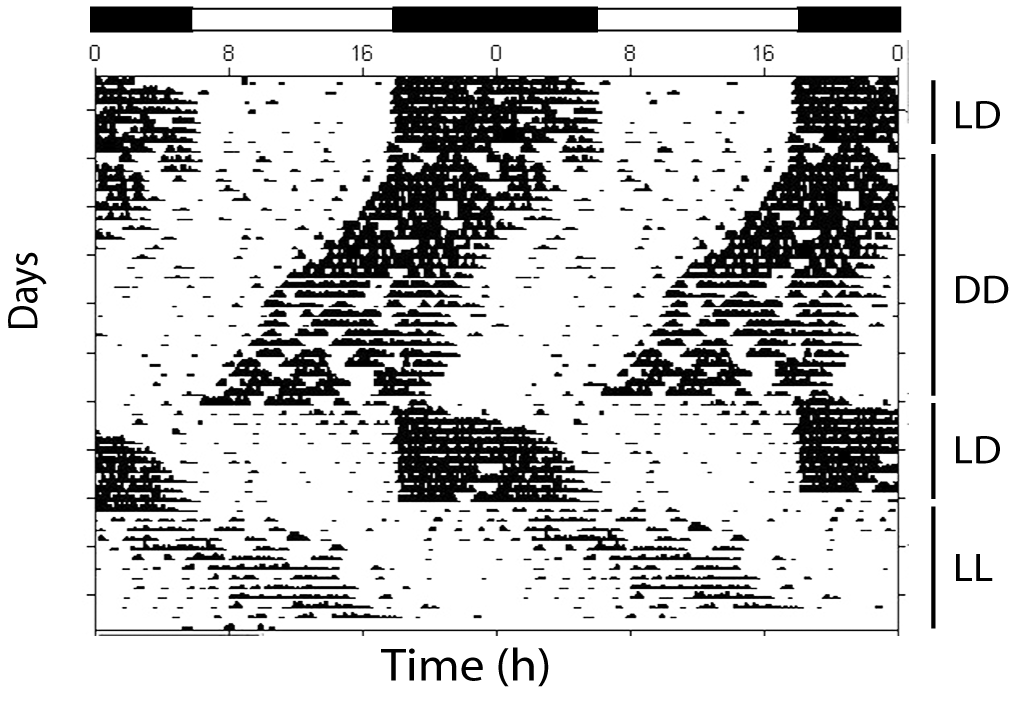
Some aspects of circadian rhythms are illustrated in Figure 1. The image is an actogram of a single mouse in a running wheel. The bars at the top show the activity of a single 24 hour period is displayed twice to assist in visualizing the rhythm as it moves.
During the first part of the record, the mouse is maintained in a light dark cycle (LD). Note where the activity falls - is this a nocturnal or diurnal animal[5]? Then note what happens when the lighting is changed to constant darkness (DD). The activity starts earlier and earlier each day. If you were to calculate the time for the onset of activity on the first day in DD and the time for the onset of activity on the second day it would be somewhat less than 24 hours - in other words - the rhythm has shortened. Notice how quickly the rhythm snaps back to the darkness when the lighting is changed to LD. While the rhythm seems to be entrained by the LD cycle, the speed of entrainment suggests that the LD cycle is masking the underlying rhythm. That is, the mouse likes the dark and has become active in the dark, overriding its underlying clock. This is somewhat similar to having a schedule that requires one to be awake earlier or later than one would like. The alarm clock (and possibly coffee) allow you to be awake at times when you would rather be sleeping. The masking is revealed in the final part of the rhythm when the lights are continuously on (LL). If you draw a line for the onset of the activity, you can see that it is aligned with where the activity starts on the last day of DD, rather than where it starts on the last day of LD. One last point - the period of the rhythm in LL is greater than 24 hours - the rhythm has lengthened.
Figure 1: Mouse wheel running activity. Each mark indicates one wheel revolution. The light dark cycle (LD) is indicated by the black bars at the top of the figure (the filled bar is darkness). the hours in each cycle are indicated by the numbers at the top of the figure. Note that the Light Dark cycle is not always present. DD indicates constant darkness ; LL indicates constant light. File:Mouse actogram Hundahl et al 2012.png. (2016, November 27). Wikimedia Commons, the free media repository. Retrieved 20:32, March 5, 2019 from https://commons.wikimedia.org/w/index.php?title=File:Mouse_actogram_Hundahl_et_al_2012.png&oldid=221247629.
Checkpoint:
Which of the following is a reasonable explanation for why circadian rhythms exist?
- We live in a rhythmic world, so circadian rhythms allow organisms to sync their internal modulation of homeostasis with the environment to be prepared and adapt in order to aid survival and reproductive success
- We live in a nonrhythmic world, so circadian rhythms give organisms an intrinsic rhythm that allows them to maintain homeostasis independent of the environment in order to aid survival and reproductive success
- We live in a rhythmic world, so circadian rhythms give organisms an intrinsic rhythm that allows them to maintain homeostasis independent of the environment in order to aid survival and reproductive success
- We live in a nonrhythmic world, so circadian rhythms allow organisms to sync their internal modulation of homeostasis with the environment to be prepared and adapt in order to aid survival and reproductive success
Answer: A
Which of the following is not a known period of biological rhythms?
- Ultradian
- Infradian
- Microseconds as shown in spontaneously firing Hindmarsh rose neurons
- Biannual rhythms as shown in the blooming of cherry blossom trees
- These are all examples of biological rhythms
Answer: E
All the choices provided portray a process that displays an endogenous, entrainable oscillation of roughly 24 hours (some a little more than 24 hours, some a little less than 24 hours) therefore they can all be classified as biological rhythms, leaving E as the correct answer choice.
Which of the following is not one of the three criteria for true biological clocks?
- Biological rhythms persist in the absence of external cues with a period close to the period they exhibit in the presence of the cues
- Biological rhythms can stop in response to external cues
- Biological rhythms can be synchronized or entrained by external cues
- Endogenously generated rhythms are temperature compensated
Answer: B
Circadian rhythms are endogenous, meaning they occur naturally within the body. Therefore, they will persist in the absence of external cues and will not be halted by external cues. This leaves B as the correct choice.
True or False: If a group of lab rats were entrained to a zeitgeber, such as light, for 18 continuous hours, the rats’ circadian rhythm and sleep cycle would eventually attune to an 18 hour pattern.
Answer: False
Organisms can be entrained to a period other than 24 hours, but there are limits. 18 hours does not fall within this range of 22 to 26 hours, so it is false.
Which of the following is a zeitgeber?
- Exercise
- Drugs
- Access to food
- All of the above are zeitgebers
Answer: D
Zeitgebers are environmental cues that regulate circadian rhythms. Exercise, drugs, food, and light are all external cues that are able to entrain an organism’s biological clock to a 24 hour light/dark cycle.
The Molecular Biology of Circadian Clocks
The genetics and molecular biology of circadian rhythms have been studied in several model organisms including some unicellular eukaryotes, fungi, plants (Arabidopsis), invertebrates (Drosophila) and mammals (mice, rats, and humans). Some remarkable similarities in mechanisms have emerged across these various groups. Let us take a detailed look at the mechanism in Drosophila.
The Circadian Clock in Drosophila
Drosophila are model lab organisms whose genome has been sequenced, allowing investigators to probe the genetic basis of several behaviors. A number of genes in Drosophila are turned on when the animal is exposed to light. These genes include: effector genes whose products mediate the animal's responses (e.g. hatching or molting) and clock genes whose products regulate the circadian clock. Two key members of this group are period (per) and timeless (tim). Activation of all of these genes requires that their promoters are bound by the protein transcription factors. These transcription factors include CLOCK, which is encoded by the gene clock (clk) and CYCLE, which is encoded by the gene cycle (cyc).
*Note: The names of proteins will be designated with capitalized Roman letters; the genes that encode them indicated in lower case italics.
The Mechanism
The PER and TIM proteins (synthesized on ribosomes in the cytoplasm) form dimers. When the concentration of these gets high enough (early evening), they dissociate and are transported into the nucleus. Here, PER binds to the CLK/CYC transcription factors, removing them from the promoters of the genes they activate; shutting off transcription. Because these genes include per and tim, the result is a negative feedback loop; that is, the product of the per gene inhibits its own synthesis (as well as that of tim). Just as, through a thru the heat of a furnace turns — through the thermostat — its own production off, a rising level of PER/TIM dimers turns off further synthesis of them.
As the level of the dimer falls, inhibition is lifted and PER/TIM activity begins anew. Clock gene expression is turned off. The time required for the different effects results in the levels of PER/TIM and CLOCK oscillating in opposite phases with a circadian (~24 hr) rhythm (figure below and this short video).
Setting the Clock - Drosophila
Even without any external cues or zeitgebers, circadian cycles persist. Without the synchronizing factors circadian rhythms tend to drift away from environmental time. Under natural conditions, the clocks are "set" (synchronized) by environmental cues. Many different factors can become zeitgebers including light, food and social cues. Light is the most effective entraining factor. In Drosophila light synchronizes the circadian rhythms like this. Light, predominantly blue light is absorbed by the protein cryptochrome (CRY). This causes an allosteric change in its conformation enabling it to bind to TIM and PER. In turn, this causes TIM and PER to break down (in proteasomes) ending their inhibition of gene transcription. If this happens when PER/TIM levels are rising (late in the "day"), it sets the clock back. On the other hand, if it happens when PER/TIM levels are declining (late in the "night"), it sets the clock ahead.
Figure 2.
The molecular mechanism that regulates circadian clocks in mammals.
The Circadian Clock in Mammals
The circadian clock in mammals resembles that of Drosophila in a number of ways with many of the participating genes being homologous. However, there are some differences (fig. 2). The transcription factors that turn on the light-induced promoters are dimers of the CLOCK protein and a protein designated BMAL1. These dimers turn on three Per genes, two Cry genes (the genes encoding cryptochrome), and hundreds of effector genes whose products control a wide variety of metabolic functions (e.g., cellular respiration, glycolysis, gluconeogenesis, lipid metabolism). The PER and CRY mRNAs are exported to the cytoplasm where they are translated. Then, the PER and CRY proteins enter the nucleus where they inhibit CLOCK-BMAL1. This turns OFF transcription of Per and Cry, and they are then degraded in proteasomes. In due course these actions allow CLOCK and BMAL1 to once again stimulate transcription of Per and Cry. Thus this negative feedback loop causes the levels of BMAL1 and PER/CRY to oscillate in opposite phases (as CLOCK and PER/TIM do in Drosophila — see figure 3 and Joe Takahashi’s video ).
Checkpoint:
Which of the following are both clock genes?
- per and tim
- per and clk
- tim and cyc
- clk and cyc
Answer: A
The genes, ‘per’ and ‘tim’ are both clock genes leaving A as the correct answer choice. Clk and Cyc are both genes that encode for the transcription factor CLOCK.
Neuroanatomy of the mammalian circadian system.
Many tissues in mammals, e.g., liver, skeletal muscle, and the beta cells of the pancreas have endogenous clocks. This means that they show oscillations in secretions or functions when cultured.
Mammalian circadian rhythms are controlled by several centers in the brain. This table highlights the many areas in the human brain that have been found to regulate circadian rhythms.
| Brain Area | Function |
| Hippocampus (HP) | Works to consolidate contextual and spatial memory from short-term to long-term memory through sleep which is regulated by circadian rhythms |
| Intergeniculate Leaflet (IGL) | Regulates circadian rhythm entrainment and phase shifting |
| Locus coeruleus (LC) | Involved in REM sleep and influenced by circadian rhythms |
| Nucleus Accumbens (NA) | Responsible for mood, motor and motivation and thought to contribute to circadian rhythms |
| Pineal Gland (PG) | Regulates production of melatonin and sleep/wake cycles which are influenced by circadian rhythms |
| Raphe nuclei (RN) | Possibly involved in sleep and alertness and thought to contribute to circadian rhythms |
| Retina (Eyes) | Part of the eye involved in photoreception and source of light input which controls circadian rhythms |
| Superior Cervical Ganglion (SCG) | Associated with fight-or-flight response and controls feedback mechanism which coordinates sleep/wake cycles and circadian rhythms |
| Suprachiasmatic nucleus (SCN) | Seat of the central clock for circadian rhythms |
| Ventrolateral preoptic nucleus (VLPO) | Involved in arousal, non-REM sleep and modulation between the two which is influenced by circadian rhythms |
| Ventral Tegmental Area (VTA) | Involved in motivated learning and thought to contribute to circadian rhythms |
Suprachiasmatic Nucleus
The SCN is the master clock.
The suprachiasmatic nuclei are clusters of cells that sit just above the optic chiasm on opposite sides of the 3rd ventricle in the hypothalamus. These cells show autonomous, circadian rhythms of activity in culture and receive input from cells in the retina that transmit information about light. Lesions in the SCN abolish circadian rhythmicity. The SCN projects to a wide variety of sources including the SCG, IGL, hippocampus, VTA to regulate rhythmicity. In addition, transplant studies suggest that at least some of the output that coordinates the body’s rhythms is humoral.
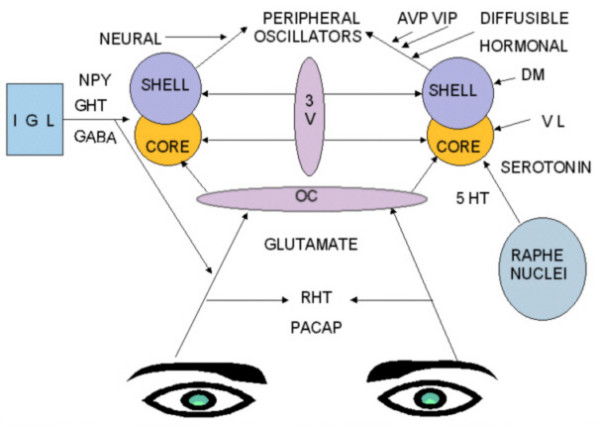
The anatomy of the SCN has been well described (fig.3). The SCN is readily divided into 2 parts: the shell and the core. The core is a collection of neurons that sit ventral and lateral (VL) just above the optic commissure. These neurons receive glutamatergic input from the retina via the Retinohypothalamic tract (RHT). The shell is a set of neurons in the dorsomedial (DM) aspect of the nucleus. The neurons in the shell utilize the neurotransmitters VIP and AVP to synchronize, entrain and shift rhythms in the body. The SCN also receivs feedback from a number of sources including the IGL and Raphe as described below.
Figure 3
. Afferent inputs and efferent pathways of the SCN. RHT: Retinohypothalamic tract, GHT: Geniculohypothalamic tract, OC: Optic chiasm, 3V: Third ventricle, IGL: Intergeniculate leaflet, DM: Dorsomedial SCN, VL: Ventrolateral SCN, NPY: Neuropeptide Y, GABA: Gamma amino butyric acid, PACAP: Pituitary adenylate cyclase-activating polypeptide.
Neuroanatomy of the SCN. Reghunandanan, V. and Reghunandanan, R., 2006. Neurotransmitters of the suprachiasmatic nuclei. Journal of Circadian Rhythms, 4, p.Art. 2. DOI: http://doi.org/10.1186/1740-3391-4-2
Retina
The retina is important in the organization and determination of circadian rhythms in retinal cells, as they are the only source of photic input into the SCN in most mammals. Cultured mammalian retinal cells exhibit an autonomous circadian rhythm in a number of neurotransmitters including: melatonin, dopamine and GABA. These cells also show circadian rhythms in clock genes, physiology and susceptibility to stress, damage and aging. Circadian rhythms in the retina are sufficient to drive rhythms in the hypothalamus in the absence of light/dark cues.
Intergeniculate Leaflet (IGL)
The SCN has an important connection with the intergeniculate leaflet (IGL). The IGL receives photic input directly from the retinohypothalamic tract (RHT), and gives rise to the geniculohypothalamic tract (GHT), which sends signals to the SCN via Neuropeptide-Y (NPY) neurons. The IGL also projects to most of the subcortical visual nuclei, indicating that the IGL receives a plethora of visual information, which possibly influences its connection with the SCN and regulation of circadian rhythms.
The IGL is known to contribute to circadian rhythm regulation through NPY signals to the SCN. In hamsters and rats, lesions in the IGL have lead to inability to entrain to certain signals, slower rates of reentrainment, and slow or no phase shift in response to stimuli. Phase advances decreased in amplitude, while phase delays increased amplitude of IGL lesioned hamsters. Although ablation of the IGL decreases circadian rhythm regulation, the lesions do not have a deleterious effect on circadian rhythms themselves like the ablation of the SCN. The clock continues to tick, but the time may vary in speed. Increases of NPY in the SCN have been observed during periods of photic transition which further supports the conclusion that the IGL regulates circadian rhythms. the IGL may also regulate the release of serotonin in the SCN. Serotonin inhibits glutamate released by the retinal ganglion cells and blocks phase advances induced by light.
It is important to note that there are differences in circadian regulation between nocturnal and diurnal, as well as anatomical differences of circadian-related structures between species. These differences may include the number and responsiveness of SCN neurons to light. Rats, nocturnal animals, and ground squirrels, diurnal animals, presented with the same light pulses exhibit peaks in NPY in the SCN at different time points indicating difference in the role of NYP in circadian rhythms differs among species.
Checkpoint:
- What is the primary type of neuron that projects from the IGL to the SCN?
- GABA neurons
- Neuropeptide Y neurons
- Serotonin neurons
- Dopamine neurons
Answer: B
Neuropeptide Y neurons are found throughout the central nervous system and are responsible for SCN input from the GHT.
2. What is the relationship between the SCN and the IGL as it relates to circadian rhythms?
- The SCN is the clock and the IGL sets the clock to the correct time and pace.
- The SCN and the IGL are the clock the clock will not run without either.
- The IGL is the clock which is set correctly on its own, but needs the SCN to keep the correct pace.
- The SCN is the clock which keeps pace on its own, but needs the IGL to set it to the correct time.
Answer: A
The SCN will continue to work following IGL ablation, but will not set to the correct time and pace.
3. Which of the following is true?
- Nocturnal and diurnal animals have NPY neuron activity in the IGL and SCN spike at different times under the same light and dark conditions.
- Although nocturnal and diurnal animals have differences in anatomy in the brain structures responsible for and controlling circadian rhythms, NYP neuron activity spikes occur at the same time as when the animals are under the same light and dark conditions.
- Nocturnal and diurnal animals have the same anatomical brain structures responsible for controlling circadian rhythms, but nocturnal animals have NYP neurons while diurnal animals do not have NYP neurons.
- There are no differences between nocturnal and diurnal animals as it relates to anatomy and NYP neurons.
Answer: A
Diurnal and nocturnal animals presented with the same light pulses both exhibit peaks in the NYP but at different times. so A is the correct answer.
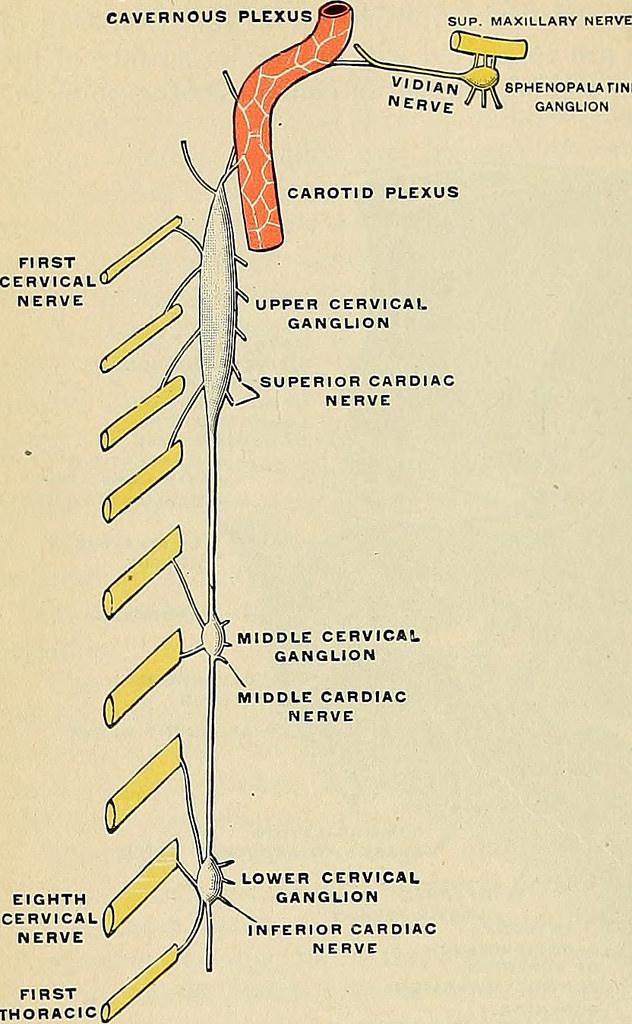
Superior Cervical Ganglion
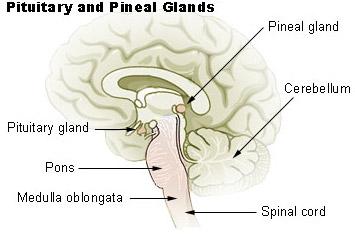
The superior cervical ganglion is part of the sympathetic nervous system and innervates both the head and neck (fig. 4). It is commonly associated with the fight or flight response. It has also been tied to circadian rhythms, as it provides sympathetic innervation to various structures in the head. Axons of the superior cervical ganglion interact with the SCN through neuron projections into the pineal gland (fig.5). The pineal gland regulates the production of melatonin, which plays an important role in circadian rhythms by regulating the sleep and wake cycles. Because of these connections, we know that melatonin metabolism is under direct influence from the SCG. Lesion studies have supported this conclusion by finding that removal of the SCG irreversibly hinders melatonin synthesis and rhythm generation. In addition, further experiments show that rats who undergo SCG removal exhibited metabolic differences compared to control groups, such as increased body weight/food intake ratio, increased adipose tissue, decreased glycemia, as well as increased daytime activity including food consumption. These findings suggest a relationship between changes in the SCG feedback mechanisms and circadian rhythm coordination.
Figure 4 (left). Anatomy of the SCG. Gray, H. (1913). Anatomy, descriptive and applied. Lea & Febiger. (right). Illustration of the pituitary gland. Pituitary & Pineal Glands. SEER Training. (2019)
Checkpoint:
- How do we know that melatonin metabolism is under influence from the SCG?
- Removal of the SCG hinders melatonin synthesis and rhythm generation
- Removal of SCG creates metabolic differences such as increased body weight/food intake ratio, increased adipose tissue, decreased glycemia, as well as increased daytime activity including food consumption
- Removal of the SCG allows the pineal gland to overcompensate, thereby producing higher levels of melatonin
- Both a and b
Answer: D
Research studies have shown that hindrance of melatonin synthesis and rhythm generation, as well as changes to metabolic systems results from removal of the SCG. Therefore, D is the correct answer.
2. The SCG is a part of which system?
- Parasympathetic nervous system
- Sympathetic nervous system
- Somatic nervous system
- Voluntary nervous system
Answer: B
The sympathetic nervous system is part of the autonomic nervous system which activates the fight or flight response, and the SCG provides sympathetic pathways to the head and neck.
3. Axons of the superior cervical ganglion interact with the SCN through neuron projections into which gland?
- Adrenal gland
- Pituitary gland
- Hypothalamus
- Pineal gland
Answer: D
Axons of the SGC innervate the pineal gland to regulate melatonin production, thereby influencing sleep and wake cycles.
Hippocampus
The hippocampus is strongly interconnected to memory consolidation through the act of sleeping. The formation of long term memories depends on reactivation of important second messenger proteins throughout the sleep cycle, which is governed by circadian rhythms in the hippocampus.
Figure 5: The effect of light, specifically sunlight, on physiology and behavior in the Suprachiasmatic Nucleus. Photo courtesy of Steve Thompson, AFMS Public Affairs http://www.jbsa.mil/News/Art/igphoto/2001481047/
Through the removal of the SCN in mice, it was found that not only circadian rhythms, but the ability to form contextual, and possibly spatial, memories was destroyed, which can be noted in the figure above. This supports the conjecture that the diurnal oscillation is not intrinsic to the hippocampus, but largely reliant on the SCN as well.
Checkpoint:
1. Which of the following statements best explains the hippocampus’s involvement in memory consolidation?
- Diurnal oscillations in the hippocampus triggers cAMP/MAPK activities, which shut down Ras activity, leading to the formation of memories.
- Circadian rhythms in the hippocampus maintain the sleep cycle, which contributes to long-term memory consolidation.
- Inhibition of hippocampal activities leads to long-term memory consolidation.
- The hippocampus has no relation to memory consolidation.
Answer: B
Circadian rhythms in the hippocampus allows for the reactivation of second messenger proteins throughout the sleep cycle, allowing for memory consolidation. Therefore B is the correct answer.
2. Which of the following best explains the relationship between the SCN and the hippocampus during diurnal oscillations?
- Diurnal oscillations are governed by both the SCN and hippocampus, which was proven through the destruction of circadian rhythms/memory consolidation upon removal of the SCN.
- Diurnal oscillations are solely dependent upon the hippocampus, which was proven when circadian rhythms/memory consolidation were unaffected upon removal of the SCN.
- Diurnal oscillations show slight dependence on the SCN, as circadian rhythms/memory consolidation were only slightly impaired upon removal of the SCN.
- The SCN and the hippocampus have no relationship in diurnal oscillations.
Answer: A
Studies in which the SCN was removed show destruction of memory as well as circadian rhythms, showing that both the hippocampus and SCN are responsible for diurnal oscillations.
Locus Coeruleus and Ventrolateral Preoptic Nucleus
The Ventrolateral Preoptic Nucleus (VLPO) is composed of two different neuronal structures: a condensed cluster of neurons, as well as an extension of neurons, both of which show heavy involvement in arousal. In studies involving lesions of the VLPO neuronal cluster, the results show a reduction in non-REM sleep. Conversely, lesions to the neuronal extension component of the VLPO show disruptions in REM sleep. The main role of this extension is to send output to the brainstem and hypothalamus. Ultimately, the Locus Coeruleus (LC), which is important in REM sleep (as well as physiological responses to stress and panic), is stimulated. The VLPO cluster, on the other hand, stimulates the histaminergic neurons, which have been found to flip the switch between arousal and non-REM sleep. During sleep, the VLPO releases GABA and galanin inhibiting neurons involved in wakefulness and arousal (i.e., LC, ORX, RN, TMN). These neurons are twice as active during REM sleep, supporting the finding of the involvement of the VLPO in REM sleep. Lesions to this brain area cause disruptions in REM sleep, which is governed by circadian rhythms.
Checkpoint:
- Which of the following is a characteristic of the Ventrolateral Preoptic Nucleus (VLPO)?
- An extension of neurons that sends output to the hypothalamus and brainstem.
- A cluster and an extension of neurons, which send outputs to varying brain areas
- A cluster and an extension of neurons, which send outputs to the same brain areas
- Both A and B
- Both A and C
Answer: D
The VLPO is comprised of a cluster of neurons, which stimulates histaminergic neurons, flipping the switch between arousal and non-REM sleep. On the other hand, the extension of neurons is known to send output to the brainstorm and hypothalamus. The key to this answer involved knowing that the VLPO consists of the cluster and extension of neurons, and that they send info to different areas.
Raphe Nuclei, Nucleus Accumbens and the Ventral Tegmental Area
Raphe Nuclei
Several nuclei - There are ongoing studies being conducted on the relationship between the Raphe Nuclei and circadian rhythms. Recent studies, involving drug treatments with BMY7378, have supported that the Raphe Nuclei regulate sleep, the timing of which is governed by circadian rhythms. The SCN influences serotonin levels involved in sleep/wake states. The Raphe Nuclei are serotonergic and maintain bidirectional connections with the SCN that may allow the SCN to influence alertness.
Nucleus Accumbens (NA)
The Nucleus Accumbens is responsible for mood, motor and motivation, and is thought to also control circadian oscillations. Interestingly, mice that display a helpless approach to stress show blunted rhythms in the accumbens when compared to their counterparts.
Ventral Tegmental Area (VTA)
The VTA is known for its involvement in motivated learning, but recent studies have shown that it may play a role in circadian regulation of sleep-wake behaviors via direct projections to the SCN. These projections utilize the neurotransmitter dopamine to influence light induced phase shifts and rentrainment.
Setting the Clock
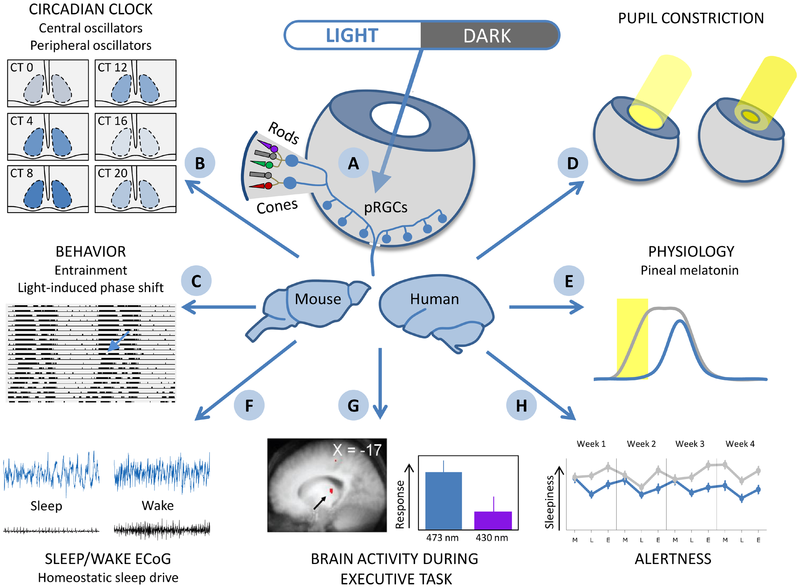
Figure 6. Summary of pervasive effects of light.
A diffuse network of photosensitive retinal ganglion cells (pRGCs), which also receive input from rods and cones, are maximally sensitive to blue light between 470 and 480 nm (A). These cells have direct connections to the central circadian oscillator in the SCN where, depending on the time of day (circadian time, CT) light induces changes in gene expression (B). pRGCs also mediate the synchronisation to LD cycles of locomotor activity, and light-induced phase shifts (C). pRGC connections to the olivary pretectal nucleus mediate light-sensitive pupil constriction (D), and indirect input via the SCN regulates the light-sensitive suppression of melatonin production in the pineal (E). The pRGC network has direct connections to sleep regulatory structures such as the VLPO and thereby modulates sleep and the ECoG during wakefulness (F). Blue light can modify brain responses to an executive task, as measured using fMRI (G), and can improve alertness (H) during the morning, lunch time, and early evening.
Source: Light, Sleep, and Circadian Rhythms: Together Again http://www.plosbiology.org/article/info%3Adoi%2F10.1371%2Fjournal.pbio.1000145
By light/dark
Light is the most powerful zeitgeber. Exposure to as little as one second of light can shift (and if repeated, entrain) rhythms of activity in nocturnal laboratory animals kept in constant darkness. There is also evidence that exposure to darkness can affect diurnal animals kept in constant darkness.
In order to set the circadian clock to light/dark cycles, perception of light is required via photoreceptors in the retina (some species have photoreceptors in the brain and skin). Without photoreceptors, normal light induced-suppression of pineal melatonin and normal circadian rhythms are disrupted. Yet, mice that were totally blind (lacking both rods and cones) had no difficulty keeping their circadian clock on time. They were able to set their rhythms because some 1–2% of the intrinsically photosensitive retinal ganglion cells (ipRGCs) in their retina, that instead of depending on signals arriving from rods and/or cones, detect light directly. These ganglion cells have an extensive network of dendrites that contain the pigment melanopsin. When exposed to light (diffuse light is fine), these ganglion cells become depolarized and send their signals back to the suprachiasmatic nucleus (SCN) as well as other brain areas involved in circadian rhythms. These ganglion cells express pituitary adenylate cyclase activating polypeptide and form the retinohypothalamic tract which signals the SCN. Although circadian rhythms will run despite the presence of specific photoreceptors, melanopsin pigment has been shown to have an important role in the mediation of photic entrainment in human circadian rhythms. Studies have shown the blue light in the range of melanopsin receptors (440- 480nm) is highly effective in phase shifting, can increase alertness, and can be used to reduce jet lag and as a treatment for seasonal affective disorder.
By food
In mice, the SCN clock, set by light/dark cycles, is the master clock as long as food is available all the time (the normal situation in the laboratory). However, if food is only offered for a few hours during the light (when mice are normally sleeping) mice shift several their behavior. For example, they begin their activity in a running wheel just before they expect food to be available. This rhythm continues even if the mice are kept in constant darkness. And it continues if the food is not provided for a day. (ref) The circadian clock that is responsible for this food entrainment is distinct from the master clock in the suprachiasmatic nucleus. Mice and rats, in which the SCN has been destroyed, continue to show food anticipatory rhythms. The dorsal striatum has been observed to regulate the food anticipatory circadian activity rhythms, specifically in mice. There have been multiple food entrainable clocks located in other parts of the brain and periphery, however, as of yet we do not know specifically where the oscillators for FAA are located.
Checkpoint
- What would happen to an individual’s circadian rhythm if a mutation was present, inhibiting PER from binding to CLK/CYC transcription factors in the nucleus?
- It would have no effect on transcription
- PER synthesis would cease, however, TIM synthesis would proceed as normal
- TIM synthesis would cease, however, PER synthesis would increase
- PER would not be able to remove the CLK/CYC transcription factors from promoters of the genes they activate, therefore, transcription would be shut off
Answer: D
CLK/CYC transcription factors can only be removed from the promoters of genes they activate via PER, therefore, if the function of PER was inhibited, removal could not be undergone and the transcription process would be halted. This leaves answer choice D as the correct answer.
- In mammals, when PER and CRY proteins enter the nucleus, they?
- Are degraded
- Further synthesized
- Inhibit CLOCK-BMAL1, thus turning off transcription of Per and Cry
- Activate CLOCK-BMAL1, thus turning on transcription of Per and Cry
Answer: C
Upon entering the nucleus, PER and CRY proteins will turn off transcription by inhibition of the CLOCK-BMAL1 gene, therefore, choice C is the correct answer.
- Which of the following is false about the SCN (suprachiasmatic nucleus)?
- It is referred to as the master clock
- It is a cluster of cells that show autonomous, circadian rhythms of activity
- It receives input from melanopsin cells in the retina that transmit information about light
- It sits above the optic chiasm on opposite sides of the 2nd ventricle
Answer: D
The SCN is a structure located in the hypothalamus that sits above the optic chiasm on either side of the 3rd ventricle. The 2nd ventricle does not exist. The notions that it is referred to as a master clock, it controls circadian rhythm activity, and it receives input from melanopsin cells in the retina are all true. This leaves D as the correct choice.
- Why are mice who are totally blind able to keep their circadian clock on time?
- Because the ganglion cells in the retina depend only on signals from rods and cones
- Because 1-2% of ganglion cells in the retina can detect light directly
- Circadian clock has nothing to do with the retina at all
Answer: B
Ganglion cells that contain melanopsin in the retina transmit information about light directly to the SCN, bypassing the need for rods and cones. This leaves B as the correct choice.
- If a mouse was unable to establish circadian rhythms to neither light nor food, what is the most likely cause?
- Two copies of its BMAL1 gene knocked out
- Two copies of its per gene knocked out
- One copy of its BMAL1 gene knocked out
- One copy of its cry gene knocked out
Answer: A
BMAL1 drives gene expression that influences circadian rhythm, so knocking out both copies would render an organism unable to establish circadian rhythms.
- What changes does a BMAL1 knockout correspond to?
- Disruptions in the brain’s circadian behavior
- Disruptions in the periphery’s metabolic properties
- Disruptions to both the brain’s circadian behavior and metabolic properties of the periphery
Answer: C
- What are the two main regulatory molecules of circadian rhythms?
- Per and Cry
- Clock and per
- Bmal1 and cry
- Bmal1 and clock
Answer: A
BMAL1/Clock are proteins that activates circadian gene expression. Per anc cry are activated by BMAL1 and Clock and can form a negative feedback loop in which the activation of BMAL1 and clock are inhibited, making them regulatory.
- What gene is responsible for the association between MDD and circadian rhythms?
- Inhibition of Clock
- Dysregulation of Cry1
Answer: B
- The farther a plant or animal lives north or south of the equator, the less pronounced the changing ratio of daytime hours to nighttime hours with the changing seasons will be.
T or F
Answer: False
This statement is false because the farther a plant or animal lives north or south of the equator, the more pronounced the changing ratio of daytime hours to nighttime hours with the changing seasons will be.
The blind and sleep
Unlike mice, people who are totally blind cannot set the clock in their SCN. As a result, their circadian rhythm drifts out of phase with the actual cycle of day and night. These people often feel fatigued during the day and wide awake when they want to be asleep at night. There is some evidence that melatonin can assist in entraining/setting the clock. However, this treatment worked only when the subject's circadian rhythm had drifted so that the normal rise in melatonin from the pineal gland was occurring in the early evening; that is, the dose of melatonin had to be given when it could boost the endogenous level of the hormone.
Clock Disorders
What is Major Depressive Disorder (MDD)?
Symptoms, Diagnosis and Treatment (Otte et. al)/(Anyan et. al) (Buoni et. al)
Mood disorders
Mood disorders such as anxiety and depression are characterized by disruptions in a variety of circadian parameters including sleep. Conversely, people with extreme diurnal preferences are more likely to also have mood disorders. This association has prompted a number of studies to identify possible connections between the circadian system and mood disorders. The results of these studies have identified interesting correlations between circadian genetics and the severity of disorders. Moreover use of bright light therapy, previously reserved for seasonal affective disorder, has produced promising results in other mood disorders (see box below).
Familial Advanced sleep-phase syndrome
Some people suffer from a disorder called familial advanced sleep-phase syndrome (FASPS). As the name suggests, it is inherited ("familial") and their circadian clocks run rapidly ("advanced"). Those afflicted tend to wake up several (up to four) hours earlier than normal. One cause of the disorder turns out to be a point mutation in the human PER2 gene. Exactly how this mutation causes shortening of the circadian cycle is still under investigation.
Seasonal affective disorder and Bright Light Therapy
Seasonal affective disorder is a mood disorder in which the symptoms vary with the changing of seasons. In the fall and winter, or the colder months, SAD is characterized by symptoms of depression, increased appetite and hypersomnia. Conversely, in warmer seasons, or spring and summer, SAD symptoms include insomnia, weight loss and moodiness. The symptoms arise at the beginning of a season and cease at its conclusion.The main cause of SAD is thought to be circadian phase delays. Because the winter brings about longer nights and shorter days, there is a delayed increase in melatonin secretion from the pineal gland. Additionally, changes in the levels of other neurotransmitters, such as norepinephrine, serotonin and dopamine, can contribute to a SAD diagnosis. Finally, alterations in the serotonin transporter and clock genes can lead to the development of SAD.
Treatment for SAD ranges from pharmacological agents to bright light therapy (BLT). BLT is a common treatment used for all depressive symptoms, either by itself or in conjunction with other psychotherapies or pharmacotherapies. BLT works by positioning a light box about 16 to 24 inches from the individual’s face. The amount of light used varies based upon the severity of the condition.Bright light therapy (BLT) has been used successfully in treating Seasonal Affective Disorder (SAD) for about thirty years. Recent applications of BLT have tested its efficacy on bipolar depression (BD). This study, conducted over a two week period, assigned thirty-two individuals with BD to treatment with either dim light or bright light for approximately thirty minutes upon waking.
It was hypothesized that the group of BD outpatients who were administered bright light for thirty minutes, daily for two weeks, would have a greater remission rate of BD than the group of BD outpatients who were administered dim light. The results for this study were measured using the Hamilton Depression Rating Scale (HAM-D) and the Montgomery-Asberg Depression Rating Scale (MADRS), and showed significantly higher rates of remission in the BLT patients. The main conclusion that can be made from this study is that BLT, when used to treat mood disorders such as BD and SAD, significantly alleviates the symptoms.(Kupeli et. al) (“About Bipolar Depression”)
Other issues with circadian rhythms:
Rhythms in Blind Persons, jet lag, shift work and puberty
Several other factors may cause an otherwise healthy person’s individual rhythms to be become desynchronized with their environment. Unlike mice, people who are blind (or lack proper photic input as in glaucoma) lack the ability to reset or entrain their circadian rhythms with light. As a result, their endogenous circadian rhythms drift out of phase with the actual cycle of day and night. Blind people may actually be awake and sleep following conventional norms and feel fatigued during the day and wide awake at night.
Jet lag is another circadian rhythm disorder. Jet lag results from travelling by air through one or more time zones. Once in the new zone the traveler may be surprised to find that they are hungry or tired at odd times. This is because, while they have arrived in a new time zone their internal clock takes several days to shift to the new rhythm.
Puberty also changes the relationship of the circadian system to the light dark cycle. While a popular view of teenage behavior suggests their inability to rise in the morning is due to late nights with friends (and social media), experiments with laboratory animals show that peripubertal rats show similar behaviors! That is there is a phase delay in circadian rhythms of post pubertal rats compared to their prepubertal behavior.
There is some evidence that melatonin can assist in entraining/setting the clock. However, this treatment only worked when the subject's circadian rhythm had drifted so that the normal rise in melatonin from the pineal gland was occurring in the early evening; that is, the dose of melatonin had to be given when it could boost the endogenous level of the hormone.
Photoperiodism
Many plants and animals not only engage in a cycle of daily activities (opening of flowers, waking, feeding, etc.) but also in seasonal activities. In plants, such things include the production of flowers and making buds dormant in preparation for winter. In animals, such things include preparing to migrate and entering and leaving hibernation. The most reliable clue to the change of season is the length of day (temperature is far less reliable!). The farther a plant or animal lives north or south of the equator, the more pronounced the changing ratio of daytime hours to nighttime hours with the changing seasons.
It is not surprising then that both plants and animals mainly depend on photoperiod to prepare for changes in seasonal activities. And what better way to measure the relative length of day and night than by enlisting the machinery by which circadian rhythms are entrained? One promising model for photoperiodic responses in plants depends on circadian rhythms. See Kimball’s Biology Pages for a discussion of this model.
As for animals, recent work with Drosophila suggests that fruit flies use two circadian clocks to monitor the changing length of day and night. The first is an "evening" clock that takes over in the long days of summer. The second is a "morning" clock that is inhibited by light but takes over when the nights are getting longer; The molecular machinery (Cry, Tim, Per, etc.) for each clock is confined to separate neurons in two different parts of the brain. Evidence for 2 clocks has also been discovered in hamsters.
In these experiments, Drosophila is using the two clocks to adapt daily — not seasonal — cycles of activity to the changing seasons. But this machinery for measuring photoperiod could enable them to prepare for seasonal changes in activity, e.g., to stop forming eggs at the end of the summer. (However, other studies examining such seasonal changes in Drosophila find that the photoperiodic response is independent of circadian responses. So, we must await more experiments to resolve the question.)
Checkpoint:
- Which of the following brain areas communicates with the SCN to signal alertness and wakefulness?
- Hippocampus
- Ventral Tegmental area
- Raphe nuclei
- Nucleus accumbens
Answer: C
- Which of the following is true regarding PER/TIM activity in Drosophila after PER/TIM inhibition of gene expression is lifted?
- When PER/TIM levels rise, the clock is set ahead.
- When PER/TIM levels rise, the clock is set back.
- When PER/TIM levels decline, the clock is set back.
- PER/TIM levels have no correlation with setting the circadian clock.
Answer: B
- Which of the following is NOT considered a cause of SAD?
- Changes in clock genes
- Circadian phase delays
- Genetic inheritance
- Varying neurotransmitter levels
Answer: C
- Select which example shows the bidirectional relationship between circadian rhythm and feeding:
- A dog will salivate when a bell is rung if conditioned to eat afterward.
- Martha always gets very hungry a few minutes after she wakes up.
- Bernadette gets hungry while watching others eat.
- Joey loves strawberries because they remind him of summer.
Answer: B
- Select one example that shows how inconsistencies in circadian rhythm can lead to metabolic disease:
- Suzy and her father were both diagnosed with diabetes at age 45.
- Harold has been overweight throughout his entire life.
- Jean has gained 40 lbs since starting to work the night shift two years ago.
- Debra was diagnosed with diabetes during the winter.
Answer: C
6. Which of the following treatments for Major Depressive Disorder is being used because of the recent discovery correlating MDD with clock genes?
- Psychotherapy
- Bright light therapy
- Pharmacological Treatment
- Exercise
Answer: B
References:
Farhud (2018) Circadian rhythm, lifestyle and health: a narrative review. Iranian journal of public health 47: 8. ISSN: 2251-6085 Online ISSN: 2251-6093
Huang W, Ramsey KM, Marcheva B, Bass J. (2011) Circadian Rhythms, Sleep, and Metabolism. J Clin Invest. 2011 Jun;121(6):2133-41. doi: 10.1172/JCI46043. Epub 2011 Jun 1. https://doi.org/10.1172/JCI46043.
Morin, L. P. (2013) Neuroanatomy of the extended circadian rhythm system. Experimental Neurology, 243(SI) 4-20
Roenneberg, T. and Merrow, M. The Circadian Clock and Human Health Current Biology 26(10) R432 R443https://doi.org/10.1016/j.cub.2016.04.011
Rosenwasser, A.M. (2009) Functional neuroanatomy of sleep and circadian rhythms. Brain Res Rev. 2009 Oct;61(2):281-306. doi:10.1016/j.brainresrev.2009.08.001. Epub 2009 Aug 18.
Singh, S.V. and Kumar, S. (2018) Circadian rhythm and their significance in relation to physiological functions of animals: A review Journal of Entomology and Zoology Studies 6(4): 1861-1866. online: http://www.entomoljournal.com/archives/2018/vol6issue4/PartAE/6-4-259-237.pdf
SunBin, Y., Postnova, S. and Cistulli, P.A. (2019) What works for jetlag? A systematic review of non-pharmacological interventions Sleep Medicine Reviews: 43 47-59. https://doi.org/10.1016/j.smrv.2018.09.005
Takahashi, J.S. (2015) Molecular components of the circadian clock in mammals. Diabetes, Obesity and Metabolism.17(S1) https://doi.org/10.1111/dom.12514
Bjorvatn B, Pallesen S. (2009) A practical approach to circadian rhythm sleep disorders. Sleep Med Rev. Feb;13(1):47-60. doi: 10.1016/j.smrv.2008.04.009. Epub 2008 Oct 8. https://doi.org/10.1016/j.smrv.2008.04.009
Sack, RL, Lewy AJ, Blood, M L, Keith, L D, Nakagawa,H (1992) Circadian rhythm abnormalities in totally blind people: incidence and clinical significance. The Journal of Clinical Endocrinology & Metabolism, 75(1) 127–134, https://doi.org/10.1210/jcem.75.1.1619000
Hagenauera,M.H., Perrymana,J.I., Leea–c. Carskadond,T.M.M.A (2009) Adolescent Changes in the Homeostatic and Circadian Regulation of Sleep, Dev Neurosci;31:276–284 DOI: 10.1159/000216538
[1] Earnest DJ, Turek FW (1983). Effect of one-second light pulses on testicular function and locomotor activity in the golden hamster. Biol Reprod Apr;28(3):557-65. https://doi.org/10.1095/biolreprod28.3.557
[2] Stephan FK, Swann JM, Sisk CL.(1979) Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behav Neural Biol. Apr;25(4):545-54. https://doi.org/10.1016/S0163-1047(79)90332-7
[3] Yamanaka Y1, Honma S, Honma K. (2013) Daily exposure to a running wheel entrains circadian rhythms in mice in parallel with development of an increase in spontaneous movement prior to running-wheel access. Am J Physiol Regul Integr Comp Physiol. Dec;305(11):R1367-75. doi: 10.1152/ajpregu.00389.2013. Epub 2013 Oct 9.
[4] Kosobud AE1, Gillman AG, Leffel JK 2nd, Pecoraro NC, Rebec GV, Timberlake W. (2007) Drugs of abuse can entrain circadian rhythms. Scientific World Journal Nov 2;7:203-12. http://dx.doi.org/10.1100/tsw.2007.234
[5] Nocturnal - the activity occurs during the dark period.