Kitchen Science: Kale Pesto and Ricotta - A pH Lab
Summary
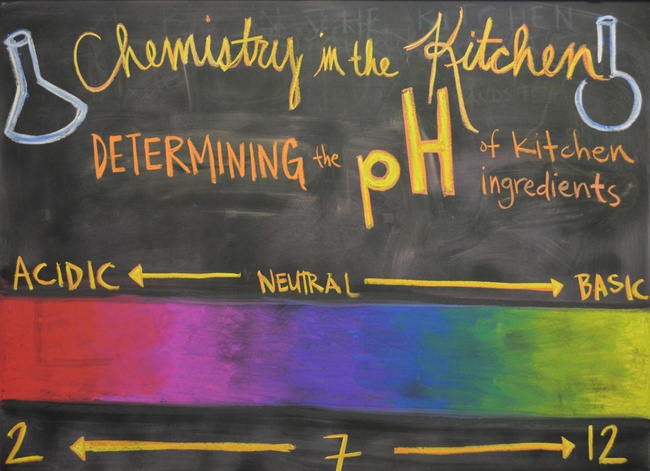
In this 8th grade science lesson, students prepare Kale Pesto and Homemade Ricotta Cheese. Students also visit the pH Lab where they use cabbage juice as an indicator to test the pH of common kitchen ingredients and products.
Objectives
After this lesson, students will be able to:
- Identify properties of acids and bases
- Identify what a high number and a low number signify on the pH scale
- Use cabbage juice as an indicator to determine whether a kitchen ingredient is acidic, basic, or neutral
Assessments
During this lesson, students will:
- Make observations and look for evidence to inform a hypothesis as to whether a kitchen ingredient is acidic, basic, or neutral
- Test an ingredient at the pH lab and approximate a number on the pH scale
- Use cabbage juice as an indicator to test whether a solution is acidic, basic, or neutral
Materials
For the Chef Meeting
- Kale Pesto recipe
- Homemade Ricotta recipe
- Ingredients and tools for demonstration
- Visual aid
Ingredients
- Baguette
• For the Kale Pesto
• Almonds
• Garlic
• Parmesan cheese
• Kale
• Lemon juice
• Salt
• Pepper
• For the Ricotta
• Whole milk
• Heavy cream or whipping cream
• Lemon juice or white vinegar
• Salt
• Pepper
• Fresh herbs (optional)
Tools
- Serrated knife
- Cutting boards
- Sheet pan
For the Kale Pesto
• Mortar and pestle
• Cast iron skillet
• Stock pot
• Spider
• Mixing bowls
• Paring knives
• Cutting boards
• Measuring cups
• Measuring spoons
• Reamer or juicer
• Cheese grater
• Rubber spatula
• For the Homemade Ricotta Cheese
• Measuring cups
• Measuring spoons
• Stock pot
• Wooden spoon
• Reamer or juicer
• Sieve or fine mesh strainer
• Clean dish towel
• Mixing bowl
Equipment
- Stove
For the pH Lab
- A variety of kitchen ingredients and products (e.g. lemons, grapefruits, oranges, vinegars, liquid soap, baking soda, laundry detergent, cream of tartar)
- A table
- White butcher paper
- Clear cups
- Measuring cups
- Measuring spoons
- Cabbage juice indicator
- Visual aid of the pH scale
Before You Begin
- Collect all the tools and ingredients, and then distribute them to the tables
- Gather supplies for the Chef Meeting
- Make the cabbage juice indicator
- Cover the table with butcher paper and draw a numerical pH scale
- Set up the kitchen ingredients and products for testing
- Create the visual aid
- Copy the Kale Pesto recipe to hand out
- Copy the Homemade Ricotta Cheese recipe to hand out
Procedures
At the Chef Meeting
- Welcome students to the kitchen and explain that cooking is chemistry. Introduce the Kale Pesto and Homemade Ricotta Cheese recipes and explain the Kitchen pH Lab.
- Review the numbers on the pH scale and how they correlate with acidity.
- Explain what an indicator is and how it works. Demonstrate how cabbage juice will be used as an indicator to determine the acidity of common ingredients and products found in our kitchen.
- Identify common characteristics of acids (taste sour, frequently liquid or gas) and bases (taste bitter, feel slippery, frequently solid). Explain that before they use the cabbage juice to test for pH, students will use their five senses to make observations and look for evidence to inform a hypothesis as to whether the kitchen ingredients and products are acidic, basic, or neutral.
- Explain that students will take a break from their cooking to visit the pH lab. Divide students into their table groups and lead one of the tables to the pH lab for the first rotation.
At the Table
- Meet with the table groups to review the recipes and assign jobs.
- Prepare the recipes and set the table. While students are cooking, have rotate through the pH lab.
- Eat.
- Clean up.
At the pH Lab
- Gather students around the pH lab table and introduce the kitchen products and ingredients students will be testing.
- Tell students to choose one of the kitchen ingredients or products to test. Using their senses to make observations, ask students to hypothesize where the ingredient or product will fall on the pH scale. If the chosen substances are edible, have students taste them to collect more evidence. Ask students to share the characteristics that informed their hypothesis (it was slippery, it was sour, etc).
- Have the students measure 1/4 cup of cabbage juice indicator and pour it into a clear cup.
- Have students mix 1 teaspoon of their kitchen ingredient or product into the cabbage juice indicator.
- Observe for color change and compare the new color to the pH scale on the visual aid. Ask students to approximate a number on the pH scale for their ingredient or product.
- Using the pH scale on the butcher paper, have students place their cup in the appropriate range.
- Back at the table, review the hypotheses and discuss the results. Ask students to explain which senses they used to collect the evidence that informed their hypothesis.
At the Closing Circle
Ask students to share the kitchen ingredient they tested and whether it was acidic, basic, or neutral.