1.2 - Measurement in Science_May
1.2 - Measurement in Science
Lesson Objectives
- Compare and contrast qualitative and quantitative observations.
- Identify the standard units of measurement within the SI System.
- Convert metric measurements from one unit to another.
Vocabulary
- International System of Units
- qualitative observations
- quantitative observations
Introduction
Measurements are a basic necessity in science. Scientists have designed thousands of different tools to help in the vital process of measuring. In this image of the control panel of the space shuttle Atlantis, we see dozens of readouts from measuring systems.

Measurement
We already know that observations are an important part of the scientific method. Hypotheses are accepted or rejected based on how well they explain observations. Some observations, such as "the plant turned brown" are qualitative observations; these observations have no associated numbers. A quantitative observation includes numbers, and is also called a measurement. A measurement is obtained by comparing an object to some standard. Any observation is useful to a scientist, but quantitative observations are commonly considered more valuable. Even if the measurement is an estimate, scientists usually make quantitative measurements in every experiment.
Consider the following pair of observations.
- When the volume of a gas is decreased, its pressure is increased.
- When the volume of a gas is reduced from 2.0 liters to 1.0 liter, the pressure increases from 3.0 atm to 6.0 atm.
A great deal more information, and more useful information, is available in the second observation.
Since accurate measurement is a vital tool for doing science, a consistent set of units for measurement is necessary. Scientists throughout the world use the International System of Units (also called the SI system). The SI system is basically the metric system, which is convenient because units of different size are related by powers of 10. The system has physical standards for length, mass, and time. These are called fundamental units because they have an actual physical standard.
The standard SI unit for length is the meter, and is denoted by "m". Originally, the meter was defined as the length between two scratches on a piece of metal which was stored in a secure vault under controlled conditions. The meter's definition has changed over time, but it is now accepted to be the distance light travels in a vacuum over 1/299792458 of a second.
The standard unit of time, the second, was once defined as a fraction of the time it takes the earth to complete an orbit around the sun, but has now been redefined in terms of the frequency of one type of radiation emitted by a cesium-133 atom. Seconds are denoted by "s" or, less commonly, "sec."
The standard unit for mass is the kilogram. The kilogram's standard is a block of platinum-iridium metal kept near Paris, France. Other countries, of course, keep copies. A kilogram is denoted "kg" and is a multiple of the smaller unit of mass, the gram ("g").
Meters, seconds, and kilograms are not the only unit entities. Take, for example, speed. Speed is a derived unit, measured in meters per second (m/s). Derived units are units that are expressed using combinations of the fundamental units.
As mentioned earlier, the SI system is a decimal system. Prefixes are used to change SI units by powers of ten. Thus, one hundredth of a meter is a centimeter and one thousandth of a gram is a milligram. The metric units for all quantities use the same prefixes. One thousand meters is a kilometer and one thousand grams is a kilogram.
| Measurement | Definition | Units |
| Length | distance from one end of something to the other end | meter (m) |
| Mass | amount of matter in an object | kilogram (kg) |
| Weight | gravitational force on an object | newton (N) |
| Area | amount of surface included within a set of boundaries | square meters (m2) |
| Volume | amount of space occupied by an object | cubic meters (m3) or liter |
| Density | amount of matter that occupies a given space(Mass / Volume) | g/cm3, g/mL, or kg/m3 |
| Time | interval between two events | second (s) |
| Temperature | average kinetic energy of the particles that make up a material | Kelvin (K)degrees Celsius (°C) |
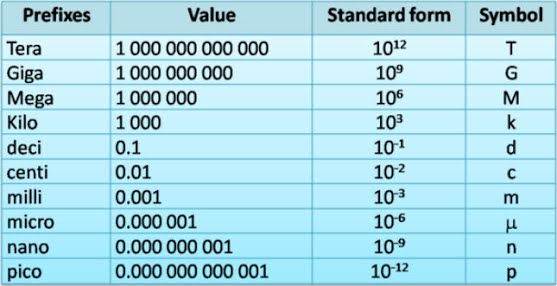
Common prefixes are shown in the table above. The same value can be represented in different ways by using prefixes. For example, 1 meter = 100 centimeters. Similarly, 0.01 meters = 1 centimeter.
Examples
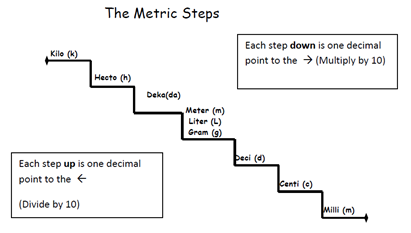
Example 1
Convert 500 millimeters to meters.
500.0 mm = ___________ m
Step 1: Count how many steps on the step ladder you need to move. Milli to meter is 3 steps to the left.
Step 2: Move your decimal the same amount of steps → 3 decimals to the left.
500.0 mm → 0.5 m
Example 2
Convert 0.437 kilogram to grams.
0.437 kg = ___________ g
Step 1: Count how many steps on the step ladder you need to move. Kilo to gram is 3 steps to the right.
Step 2: Move your decimal the same amount of steps → 3 decimals to the right.
0.437 kg → 437.0 g
Lesson Summary
- Measurements (quantitative observations) are often more useful than qualitative observations.
- The system of units for measurements in science is the SI system.
- The fundamental quantities in the SI system are length, mass, and time.
- The SI unit for length is the meter, for mass is the kilogram, and for time is the second.
- Prefixes are used to change SI units by powers of ten.
Lesson Review Questions
- Which of the following are quantitative observations?
- The sky is blue.
- The toy car is about 3 inches long.
- It is 250,000 miles from the earth to the moon.
- The wooden cart has a mass of 18.654 g.
- When at rest, the pendulum points toward the center of the earth.
- Convert 76.2 kilometers to meters.
- Convert 76.2 decigrams to kilograms.
- Convert 1 day into seconds.
Sources:
https://sites.google.com/site/vccsengineeringbridgemodules/engineering-units